505(b)(2) Drug Development Trends
There are many reasons to consider the 505(b)(2) approval pathway when developing new therapeutic topical and transdermal products that utilize known active pharmaceutical ingredients.
While Tioga Research is focused exclusively on supporting clients interested in the research and early development of topical and transdermal formulations, there are common elements to the 505(b)(2) process, regardless of dosage format, that are interesting to consider.
The information below is taken from two recent articles (see References) reviewing the increasing use of the 505(b)(2) pathway and detailing the benefits and opportunities, both from patient healthcare and commercial perspectives, provided by this drug development pathway.
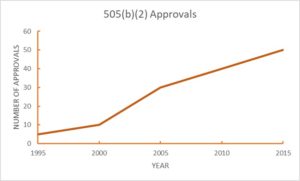
While the 5050(b)(2) pathway was made available in 1984 (Hatch-Waxman Act) it was not until the mid-1990’s that it started to be utilized; and its use has grown considerably since. To illustrate this development it is interesting to note that for the last 15 years there have been more 505(b)(2) approvals each year than 505(b)(1) new molecular entities (NME) approvals.
More than 40 drug products are approved each year via the 505(b)(2) pathway. Of the over 600 drugs approved via this pathway between 1993 and 2016, many had market exclusivity ranging from 3 to 7 years. In future postings we will interrogate these data further and consider the types of products approved, the review process, product formats, and patient indications that have benefited from the 505(b)(2) approval pathway.
References
“The 505(b)(2) Drug Approval Pathway” by Jonathan J. Darrow, Mengdong He and Kristina Stefanini in the Food and Drug Law Journal (2019)
“Review of Drugs Approved via the 505(b)(2) Pathway: Uncovering Drug Development Trends and Regulatory Requirements” by Ingrid Freije, Stephane Lamouche and Mario Tanguay in Therapeutic Innovation & Regulatory Science (2019)

Leave a reply